Tumor immunotherapy based on the interconnected use of innate and acquired immunity
Despite significant progress in cancer therapy, everything is far from being resolved. It is reported that every third person gets cancer and every fourth person dies from it. Between 2035 and 2050, cancer is expected to become the leading cause of death in the U.S.
In the last decade, attention in the field of cancer therapy has been increasingly focused on the possibility of using patients’ own immune systems to fight tumors. The use of checkpoint inhibitors has become significant, for which the Nobel Prize was awarded in 2018. The mechanism of action of these inhibitors is often compared to the release of brakes, in this case the feedback brakes of the immune system. One danger is evident, and that is that a inhibited immune system will cause damage. This often happens in the form of induction of autoimmune diseases. On the other hand, this strategy can only work if there is a primary immune response and it is only strengthened by removing the brakes. In the spirit of the above analogy, it can be said that a car standing on a flat surface will not start moving if we release the brakes. As a result, immunotherapy based on checkpoint inhibitors only works in a limited number of diagnoses and only in about 20% of cases.
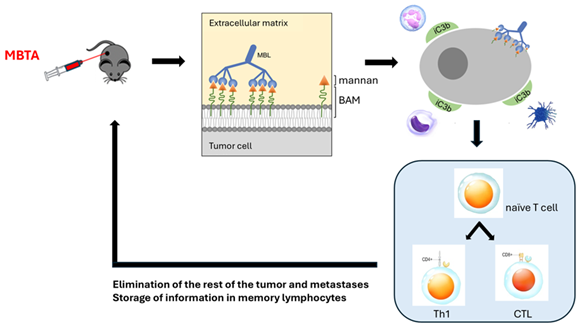
In our laboratory under the Department of Medical Biology of the Faculty of Science of the University of South Bohemia in České Budějovice, we decided to choose a different approach. Instead of releasing feedback brakes on the final attack of tumors at the level of acquired immunity, we tried to imitate the overall natural process of eliminating dangerous agents. When a pathogenic bacterium enters the body, for example, a cascade of reactions is set in motion. Cells of the first immune line of defense, i.e. cells of innate immunity, using their Pattern Recognition Receptors (PRRs), are able to recognize these pathogens because they detect the so-called Pathogen Associated Molecular Patterns (PAMPs). Of course, the relevant molecules do not occur on tumor cells. Therefore, the idea arose to artificially place these motifs on tumor cells and thus fool innate immunity. A number of such possibilities were successfully detected, and in the end, mannan, a component of yeast walls, was selected. As a result, for innate immune cells, tumor cells labeled in this way look like yeast, so they are attacked. Phagocytes (neutrophils, macrophages, dendritic cells) play an important role in this attack, but natural killers, NK cells, are also involved.
The key part of the therapy is therefore mannan. During a simple synthesis, we bind a biocompatible anchor for membranes, the BAM compound, to it. This anchor ensures that the tumor cells are enveloped with mannan. However, this alone is not enough. It is necessary to add several more compounds from the above-mentioned group of PAMPs, namely resiquimod, poly (I:C) and lipoteichoic acid. Their task is to attract innate immune cells to the tumor. This was enough to eliminate some tumors, but it turned out that for more resistant tumors and especially for metastatic tumors, it is necessary to add one more component, namely the anti-CD40 antibody. It helps to involve the second component of immunity, acquired (adaptive) immunity, in the elimination of tumors. It then not only destroys the remnants of the tumor, but also attacks metastases and stores everything in memory, so it quickly intervenes in the event of recurrences of the disease (Fig. 1).
The immunotherapeutic described above is called MBTA. This immunotherapeutic agent is mainly applied intratumorally. Its application leads to effective elimination of tumors in mouse models of melanoma, sarcoma, pancreatic adenocarcinoma, colorectal cancer, pheochromocytoma, breast cancer and glioblastoma in our laboratory and in the laboratories of Professor Pacák and Professor Zhuang at the NIH, Bethesda, Maryland. Very good results (87.5% cure) were also achieved in the case of pancreatic adenocarcinoma, which is otherwise very difficult to treat. Checkpoint inhibitors, currently considered the “gold standard of immunotherapy”, did not work at all.
If minor metastases are present, the body vaccinated with its own tumour can reliably eliminate them. In the case of large metastases, the best solution is also to inject MBTA. Up to two-thirds of mice can be cured in this way. If this is not feasible, it has proven appropriate to apply MBTA to an accessible tumor (it can also be a large metastasis) and to combine this immunotherapy with systemically administered substances that work in synergy. We have successfully tested the synergy of MBTA with inhibitors of glutamine metabolism and with substances inducing apoptosis of tumor cells. Combination with checkpoint inhibitors proved to be ineffective.
Figure 1: Schematic representation of MBTA mechanism